|
|
 |
The Planck Function
A theoretical explanation for cavity radiation was the
single most compelling unsolved problem in physics at the beginning of
the twentieth century. The best fit to observations had been suggested
by Wein:
-
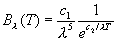
where c1 and c2
were determined experimentally. In October 1900 Max Planck proposed a small
modification to this relationship, namely
-
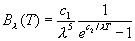
Planckís relationship, now called the Planck function, was
a better fit to the data but was not an explanation for why radiation behaves
this way. To develop a theoretical model, Planck imagined that the atoms
in the cavity walls behave like tiny oscillators, each with its own characteristic
frequency, each emitting radiation into the cavity and absorbing energy
from it. But it proved impossible to derive the properties of cavity radiation
until he made two radical assumptions:
-
that the atomic oscillators cannot take on arbitrary
values of energy E. Instead, the oscillators have only values of
E that satisfy
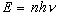 where n is an integer called the quantum number, n
the oscillator frequency, and h a constant.
where n is an integer called the quantum number, n
the oscillator frequency, and h a constant.
-
that radiation occurs only when an oscillator changes
from one of its possible energy levels to another; that is, when n
changes value. This implies that the oscillators cannot radiate energy
continuously, but only in discrete packets, or quanta.
By December of 1900 Planck had worked out a complete theory
for cavity radiation, including the values of c1 and
c2:
-
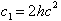
-
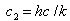
In these equations k is Boltzmannís constant,
which appears in statistical mechanics and thermodynamics, and h
is Planckís constant. Planck used observations of 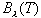 to determine the values
to determine the values 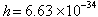 J
s and J
s and 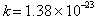 J K-1. J K-1.
Next
Back
Return to Lesson 2
Return to Satellite Meteorology Main Page
|



