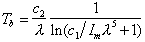The radiation emitted by any object can be related to
the blackbody radiation
-
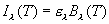
where el is
the emissivity of the object, which varies between 0 and 1. If el
does not depend on wavelength we say that the object is a gray body; a
blackbody has a value of el=1.
How are emission and absorption related? Imagine an object
that absorbs perfectly at one wavelength but not at all at any other wavelength
(that is, an object with el =1 at
one value of 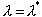 and el=
0 everywhere else), illuminated by broadband blackbody radiation from a
second body. The object absorbs the incident radiation and warms; as it
warms the emission (which occurs only at l?,
remember) increases. Equilibrium is reached when the amount of energy emitted
by the particle Eout is equal to the amount absorbed
Ein. At wavelength l?
the body acts as a blackbody, so emission depends only on the equilibrium
temperature and
and el=
0 everywhere else), illuminated by broadband blackbody radiation from a
second body. The object absorbs the incident radiation and warms; as it
warms the emission (which occurs only at l?,
remember) increases. Equilibrium is reached when the amount of energy emitted
by the particle Eout is equal to the amount absorbed
Ein. At wavelength l?
the body acts as a blackbody, so emission depends only on the equilibrium
temperature and 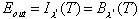 . The
body is exposed to broadband blackbody radiation, so
. The
body is exposed to broadband blackbody radiation, so 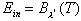 .
But since equilibrium implies that
.
But since equilibrium implies that 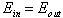 ,
the absorption at every wavelength other than l*
must be zero. This chain of reasoning, known as Kirchoff’s
Law, tells us that the absorptivity and emissivity of objects is the same
at every wavelength.
,
the absorption at every wavelength other than l*
must be zero. This chain of reasoning, known as Kirchoff’s
Law, tells us that the absorptivity and emissivity of objects is the same
at every wavelength.
The Planck function finds another application in the computation
of brightness temperature. If we make measurements of monochromatic intensity
Im at some wavelength l, and
assume that el =1, we can invert
the Plank function to find the temperature Tb at which
a blackbody would have to be in order to produce the measured intensity
-